Is Limitation of Hip Abduction a Useful Clinical Sign in the Diagnosis of Developmental Dysplasia of the Hip?
Q Choudry, R Goyal, R W Paton
Arch Dis Child. 2013;98(11):862-866.
Abstract and Introduction
Abstract
Aim The relationship between the presence and severity of sonographically diagnosed developmental dysplasia of the hip (DDH) and the clinical abnormality of limitation of hip abduction (LHA) was investigated.
Methods A prospective, longitudinal, selective 'at risk' and neonatal instability hip ultrasound programme between 1 January 1996 and 31 December 2005. 2876 neonates/infants were initially screened for DDH by clinical examination and by hip ultrasound imaging. Pathological sonographically evaluated DDH was considered to be Graf Type III, IV and irreducible hip dislocation. Inclusion criteria were cases of unilateral or bilateral limitation of hip abduction hip. Exclusion criteria: syndromal, neuromuscular and skeletal dysplasia cases.
Results 492 children presented with LHA (55 unilateral LHA). The mean age of neonates/infants with either unilateral or bilateral LHA was significantly higher than those without (p<0.001). In the sonographic diagnosis of Graf Type III and IV dysplasias, unilateral LHA had a PPV of 40% compared with only 0.3% for bilateral LHA. The sensitivity of unilateral LHA increased to 78.3% and a PPV 54.7% after the age of 8 weeks for Graf Types III, IV and irreducible hip dislocation.
Conclusions This study identifies a time-dependent association with unilateral LHA in the diagnosis of 'pathological' DDH after the age of 8 weeks. The presence of bilateral LHA in the young infant may be a normal variant and is an inaccurate clinical sign in the diagnosis of pathological DDH. LHA should be actively sought after 8 weeks of age and if present should be followed by a formal ultrasound or radiographic examination to confirm whether or not the hip is developing in a satisfactory manner.
Introduction
The relationship between the presence and severity of sonographically diagnosed developmental dysplasia of the hip (DDH) and the clinical abnormality of limitation of hip abduction (LHA) of the affected hip is currently controversial.[1] It is generally accepted that LHA is associated with irreducible or late diagnosed dislocation.[2] There may be difficulty in detecting this clinical sign accurately.[3] Terjesen felt that LHA was the most important clinical sign of a pathological hip in DDH.[3] Stoffelen et al [4]was of the opinion that there was a link between LHA and hip dysplasia.
In a previous 5-year prospective longitudinal observational study, we suggested that if unilateral LHA is detected clinically, at 3–4 months of age, further investigation is warranted.[1] By contrast, it was noted that the presence of bilateral LHA was a poor clinical sign due to its lack of sensitivity. In this previous study, positive predictive value (PPV) was not calculated and there was a lack of data for 14% of the cases in the series, weakening its accuracy and value.
This 10-year longitudinal observational study investigated the relationship and development of LHA with DDH as demonstrated by ultrasound imaging and/or radiographs (if irreducible). It has the advantage of increased numbers and of better data collection and analysis than the previous 5-year study.[1]
Patients and Methods
Between 1 January 1996 and 31 December 2005, a prospective, observational longitudinal, targeted (clinical instability and 'at risk') hip ultrasound and clinical screening programme was undertaken at Blackburn Royal Infirmary. Neonates referred with clinical instability (as defined by a positive Ortolani or Barlow manoeuvre) were assessed by ultrasound within 1–2 weeks of age while infants considered to be 'at-risk' were assessed between 6 and 9 weeks of age. Other cases could be referred at any time from the community, usually after the 6-week general practitioner 'hip check' for clinical instability or limited hip abduction. The majority of referrals were in a bimodal distribution (within 1–2 weeks and at between 6 and 9 weeks). 'At risk' factors included a strong family history, breech presentation, postural and fixed foot abnormalities, oligohydramnios and torticollis.[5] Infants presenting later (usually referred from the general practitioner) with LHA were assessed clinically and with sonographic and/or radiographic hip joint imaging (depending on age of presentation).
During the 10-year study period, 2876 (7.66% of the birth population) neonatal instability and 'at-risk' patients were assessed by the senior author (RWP), clinically and ultrasonographically, from a total of 37 518 births from the surrounding districts of Blackburn, Hyndburn and the Ribble Valley. The clinical assessment of hip abduction was made with the patient supine with both hips flexed to 90°. The prone method was not used as the senior author (RWP) found it was difficult to fix the pelvis, making accurate assessment poor. The clinical examination was undertaken independently of the ultrasound hip scan. LHA was noted at this initial assessment, and any block to full abduction was noted from the horizontal and was considered clinically present if it was estimated to be equal to or more than 20° compared to the other hip, as less than this has been shown to be within the normal range.[1,6] Clinically, it is difficult to detect a difference between both hips in unilateral LHA of less than 20°; however, no assessment was made regarding the absolute degree of limited abduction and dysplasia as this sign was considered as positive if >20° or negative if <20°. Inclusion criteria were cases of unilateral or bilateral limitation of hip abduction hip (excluding syndromal, neuromuscular and skeletal dysplasia cases).
Ultrasound examinations were undertaken with the patient in the lateral decubitus position, with the hips flexed and adducted, in order to potentially minimise errors produced by pelvic obliquity. Static[7] and dynamic[8] (incorporating the Barlow's dynamic manoeuvre to demonstrate instability) sonographic hip imaging methods were used and the Graf α angle, measuring the osseous development of the acetabular roof, was recorded. The Graf α angle is the angle subtended by two lines, a baseline running tangentially to the wall of the ilium and an acetabular roof line. A modification of these sonographic measurements[1] was used (Figure 1).
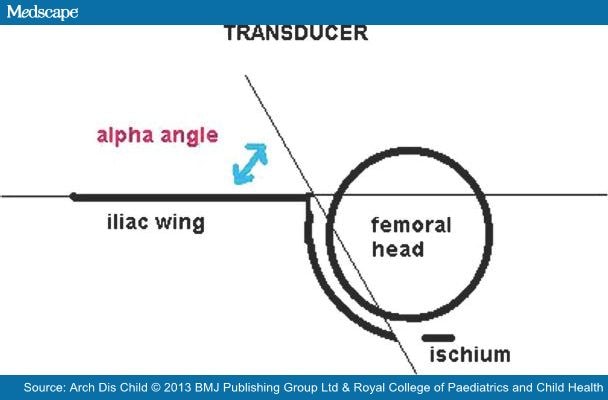
Figure 1.
Illustration of the α angle denoting the slope of the bony acetabulum. A modified Graf classification used; Type I (normal) α angle >60°, Type II α angle 43–59°, Type III α angle <43°, Type IV subluxable, dislocated dislocatable hip.
'Pathological' sonographic DDH was considered to be Graf Type III, IV or hip joint irreducibility.[1,5] As 90% of Graf Type II dysplasia spontaneously resolve, the majority of Graf Type II hips were considered to be 'physiological'.[9–11] All Graf Type II hips were followed up until normal or treated if they deteriorated. If the sonographic hip dysplasia deteriorated, the worst classification was recorded in the data analysis (ie, Graf Type III rather than Graf Type II).
Any infant, who presented after the age of 4–6 months with LHA, was initially assessed with a plain pelvic radiograph (although the hip was in addition imaged sonographically if the child was below 40 weeks old). In these cases, the diagnosis of pathological hip dysplasia was made using the radiological maturation curve of Tonnis[12] in which a pathological hip dysplasia was diagnosed if the acetabular index was below two SD of the mean for that age (the lowest 2.5% of the population).
The study was not completed until 4 years after 31 December 2005, in order to identify other cases of irreducible hip dislocation or 'pathological' dysplasia requiring surgery which were born within the 10 years of the study but which presented to the clinic at a later date (ie, 'late' cases).
Statistical analysis was conducted using XLstat comparing the three main groups of patients, namely unilateral LHA, bilateral LHA and no LHA to determine the sensitivity, specificity of the tests as well as the PPV and negative predictive values (NPV) of LHA.
Results
Limited Hip Abduction
A total of 2876 patients were examined clinically and with hip ultrasound imaging over the study period. Of these, 492 patients (17%) had LHA with 2384 patients (83%) being normal with no limitation of hip abduction on the initial clinical assessment. The sonographic hip imaging results are presented in Figure 2.
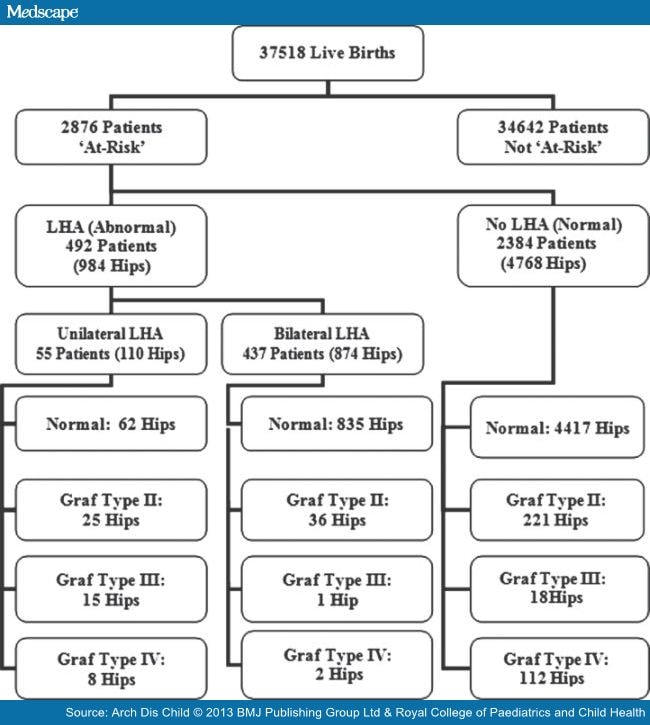
Figure 2.
Algorithm of limited hip abduction (LHA) and the ultrasound hip imaging results.
In the total sample of the 'at risk' or unstable hips (5752 hip joints), initial ultrasound showed that 5314 (92.4%) were normal, 282 (4.9%) were Graf Type II, 34 (0.6%) were Graf Type III and 122 (2.1%) were Graf Type IV. Of the 122 Graf Type IV hips (92 patients), 8 (6.6%) were in the unilateral LHA group (all on the clinically abnormal hip), 2 (1.6%) were in the bilateral LHA group (two different patients each having one hip affected) and the remaining 112 (91.8%) in the no LHA group. There were 62 patients with unilateral Graf Type IV and 30 with bilateral Graf Type IV hips (subluxation or dislocation).
The mean age of patients (regardless of the presence or absence of dysplasia and including those presenting late) were unilateral LHA group 131.5 days (95% CI 108.3 to 154.7 days), bilateral LHA group 79.1 days (95% CI 75.6 to 82.5 days) and no LHA group 44.2 days (95% CI 39.9 to 49.7 days). The difference between the ages in the unilateral and bilateral LHA group and the no LHA group was significant (p<0.0001).
If unilateral LHA is only assessed with cases of 'pathological' DDH presenting after the age of 8 weeks (Graf Type III, IV and irreducible hip dislocation), the sensitivity was 78.3%, the specificity was 92.9%, the PPV was 54.7% and the NPV was 97.3%. As the diagnosis of sonographic 'pathological' DDH is likely to have fewer false positive and negative cases, these results are likely be more accurate than if all sonographic dysplasias are assessed in the analysis.
In the no LHA group, there were 82 patients (52 unilateral and 30 bilateral) with 112 Graf Type IV hips (mean age 11.3 days 95% CI±8 days). All the Graf Type IV hips were 'early' dislocatable (within weeks) with only two patients (two hips) requiring later open reduction for failed conservative treatment in a Pavlik harness.
In the bilateral LHA group, there were two patients each presenting with one Graf Type IV hip.
As a clinical screening test the sensitivities, specificities, PPVs and NPVs for unilateral and bilateral LHA as well as the values for unilateral LHA according to age are shown in .
Table 1. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for unilateral and bilateral LHA and dysplasia of the hip
| Dysplasia | Sensitivity % | Specificity % | PPV % | NPV % |
|---|
Unilateral LHA
Type III, IV (n=23) | 14.4 | 99.3 | 40 | 97.3 |
Bilateral LHA
Type III, IV (n=3) | 2.2 | 41.1 | 0.3 | 81.8 |
Unilateral LHA
Type III, IV
Age <8 weeks (n=0) | 0 | 97.5 | 0 | 73.6 |
Unilateral LHA
Type III, IV
Age >8 weeks (n=23) | 78.3 | 92.9 | 54.7 | 97.5 |
In this 10-year study, the overall dislocation rate requiring open reduction was 0.53–0.59 per 1000 (20 infants/22 hips) and including the surgical treatment of persistent pathological radiological dysplasia the rate was 0.69 per 100 (26 cases). The two bilateral irreducible hip dislocations (1:18 759) both presented after the age of 11 months. Ninety-five per cent of the irreducible hips were identified in females (19 out of 20), whereas in late dysplasia requiring surgery, the 83.34% were in males (5 out of 6). Surprisingly of the 20 irreducible hips, 40% (8 cases) were diagnosed before 3 months (of age), 20% (4 cases) at 3–6 months, 5% (1 case) at 6–12 months and 35% (7 cases) between 12 and 20 months. No cases of irreducible dislocation were diagnosed after 20 months of age.
Discussion
Despite a paucity of evidence supporting its value in improving outcomes, universal clinical screening for DDH, with or without the addition of selective or universal ultrasound hip imaging, remains a well-established method for its detection.[13,14]
Klisic[15] described DDH as a range of hip disorders ranging between dysplasia and irreducible dislocation. This is a dynamic condition in which there can be resolution or deterioration of hip instability or dysplasias. Klisic's[15] definition of DDH includes physiological (or immature) dysplasias (Graf Type II) that generally resolve without treatment. This has resulted in an inconsistency in the literature, as some studies include Graf Type II dysplasias as pathological resulting in high diagnostic and treatment rates of 5–7%. The term DDH does not include neurological, syndromal or neuromuscular cases.[16]
The lack of a practical confirmatory 'gold-standard' clinical or sonographic diagnostic test coupled with a consistent lack of 'known' risk factors in between 69% to 73% of cases makes identification of pathological DDH difficult.[5,17] Clinical examination using provocative tests such as the Ortolani or Barlow manoeuvres continue to be the mainstay of initial diagnosis.[18] These clinical tests are not reliable enough to be considered to be an ideal screening method and should be considered surveillance.[19] Within 1 month of age, 88% of clinically diagnosed neonatal unstable hip joints may spontaneously stabilise.[20] There are very little reliable data on the natural history of Graf Type III hip dysplasia. However, some Graf Type III hips may spontaneously resolve.[9] If this is added to the vague definition of DDH,[18] there is some doubt on what constitutes 'true' pathological DDH. This makes research into this subject difficult and possibly subjective. Some consider the only 'true' pathology in DDH is the rate of irreducible dislocation of the hip.[5] The rate of irreducible hip dislocation in this study is consistent with the best universal clinical or selective screening programmes.[5,21] There is no internationally agreed definition of 'pathological' DDH. However, the general consensus is that in neonatal and infant DDH Graf Type III, IV and irreducible hip dislocation should be treated as pathological.[1,5] Screening for 'pathological' hip dysplasia is not possible as the true incidence or prevalence of this condition in the adult is unknown. The relationship between adult acetabular dysplasia (diagnosed radio logically) and neonatal hip dysplasia (diagnosed sonographically) is unknown. Although up to 30% of total hip replacements are thought to be related to adult hip dysplasia,[22,23] there is no direct proven relationship with neonatal sonographic acetabular abnormalities.
Additional physical signs previously considered to be associated with DDH, including asymmetrical thigh and gluteal skin folds, have little value in diagnosis.[14,15,20,24]
The numbers (%) of 'pathological' sonographic hip dysplasias and/or hip instability from this study are within the accepted range published within the literature.[25] The relationship between LHA and DDH remains controversial. Previous reports have suggested a link with worsening limitation,[4] less severe dysplasia[26] or even dysplasia in the contralateral hip.[27] The definition of LHA may be vague, and like the Ortolani/Barlow manoeuvres, a positive or negative result may suffice in these rather subjective tests. The sonographic hip imaging and clinical hip examinations was undertaken by the same examiner. This increases the likelihood of unintentional bias though this was unavoidable in this study as the clinic was Consultant based (RWP). The senior author, however, has over 20 years experience, and his ultrasonography image quality and interpretation have been independently validated as accurate. Selective 'screening' for DDH (clinical examination combined with hip sonography) is unusual with few orthopaedic practitioners undertaking this in the UK. As in Ortolani/Barlow manoeuvres, the experience of the examiner may be an important factor in the accurate diagnosis of LHA. Irritable infants can make examination difficult or allow for inaccurate recording in inexperienced hands.
While LHA has been identified to be present in DDH, the onset of the sign has not been accurately identified. In our previous study,[1] we noted a low level of LHA in the 'early' dislocatable (5.9%) group compared with those that presented late (87.5%) or early irreducible group (100%). Neonatal laxity is likely to be the reason for the absence of LHA in the neonate.[28] The lack of LHA in the newborn in DDH should be differentiated from the so-called moulded baby syndrome in which LHA is present at birth along with pelvic obliquity, scoliosis, plagiocephaly and postural foot abnormalities. In this condition while LHA is present at birth, there is no association with DDH and ultimately no further treatment is required for the hips.[29,30]
Sixty per cent of the irreducible hip dislocations presented after the age of 3 months. This confirms the failure of universal neonatal and 6-week General Practitioner (GP) clinical screening guidelines in addition to selective at risk sonographic hip screening in identifying and preventing the majority of irreducible hip dislocations.
Our study has shown that the sensitivity of this test was low when all types of sonographic dysplasia are included in the analysis, suggesting that LHA is not exclusive to 'pathological' DDH. Its value, however, markedly increased after the age of 8 weeks, which may also be the time that neonatal laxity itself diminishes. This is 4 weeks earlier than the accepted time frame when LHA becomes reliable.[6] While not commenting upon the precise mode of onset of LHA, other study has reported similar sensitivities of unilateral LHA (69%) in a population of infants over the age of 3 months.[31] Assessing an asymmetrical response to range of movement is easier than a symmetrical one and may account for the very low sensitivity of bilateral LHA and DDH.
We do not consider bilateral LHA in an infant <8 weeks of age to be an accurate clinical sign in the diagnosis of pathological DDH. Bilateral LHA can be rarely associated with bilateral hip dislocations; however, from a cohort of over 37 000 patients over 10 years, we have had only two cases of bilateral hip dislocations both of whom presented late. It is clearly implausible to justify effective clinical screening for such a rare condition. On the rare occasion where a walking infant presents with a severe bilateral LHA in association with postural or gait abnormality (ie, an increased lumbar lordosis and a waddling Trendelenburg gait), it could signify serious hip pathology and a pelvic radiograph should be arranged and assessed.
The high sensitivity and specificity of unilateral LHA after 8 weeks of age suggests that it is an important clinical sign which should be actively sought. This may be particularly true for the secondary general practitioner/health professional (primary healthcare) clinical hip screening programmes which recommend clinical hip screening at 6–8 weeks of age.[18,32] Detection may allow earlier intervention and potentially reduce the rate of 'late' irreducible hip dislocation presentation. Training into the importance of unilateral LHA presenting after the age of 6–8 weeks of age should be emphasised. Ideally, the clinical examination should be undertaken by small groups of experienced clinicians as wide groups of inexperienced clinicians may miss this important clinical sign. Such experienced small groups have been shown to improve effectiveness of accurate diagnosis in neonatal hip instability in Sweden.[33]
The current recommended 6-week assessment may be too early and may miss the development of the LHA. Delaying the assessment until 8 weeks may be advantageous. This change would require a proper multicentre controlled comparative trial between the different timings (6 weeks vs 8–12 weeks), showing a clear advantage before any change in timing could be recommended. Due to the low incidence of 'pathological' DDH, this series would take many years and would be difficult to organise.
In conclusion, this study identifies a time-dependent association with unilateral LHA in the diagnosis of 'pathological' DDH after the age of 8 weeks. The presence of bilateral LHA in the young infant may be a normal variant in the majority and is a poor screening sign for determining the presence of pathological DDH due to its low sensitivity and poor PPV. LHA should be actively sought after 8 weeks of age and if present should prompt for referral to a specialist (Paediatric Orthopaedic Surgeon or expert hip sonographer) followed by a formal ultrasound or radiographic examination to confirm whether or not the hip is developing in a satisfactory manner.
References
Jari S, Paton RW, Srinivasan MS. Unilateral limitation of abduction of the hip. A valuable clinical sign for DDH? J Bone Joint Surg[Br] 2002;84B:104–7.
Godward S, Dezateux C. Surgery of congenital dislocation of the hip in the UK as a measure of outcome of screening. MRC Working Party on Congenital Dislocation of the Hip. Medical Research Council. Lancet 1998;351:1149–52.
Terjesan T. ULtrasound as the primary imaging method in the diagnosis of hip dysplasia in children aged <2 years. J Pediatr Orthop 1996;5:123–8.
Stoffelen D, Urlus M, Molenaers G, et al. Ultrasound, radiographs and clinical symptoms in developmental dislocation of the hip: a study of 170 patients. J Pediatr Orthop B 1995;4:194–9.
Paton RW, Hinduja K, Thomas CD. The significance of at-risk factors in ultrasound surveillance of developmental dysplasia of the hip. J Bone Joint Surg [Br]2005;87B:1264–6.
Homer CJ, Balz RD, Hickson GB, et al. American Academy of Pediatrics, Clinical Practice Guideline: Early Detection of Developmental Dysplasia of the Hip. Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. Pediatrics 2000;105:896–905.
Graf R, Tschauner C, Klapsch W. Progress in prevention of late developmental dislocation of the hip by sonographic newborn hip screening—results of a comparative follow-up-study. J Pediatr Orthop B 1993;2:115–21.
Harcke HT, Clarke NM, Lee MS, et al. Examination of the infant hip with real time ultrasonography. J Ultrasound Med 1984;4:131–7.
Castelein RM, Dauter AJ, deVlieger M, et al. Natural history of ultrasound hip abnormalities in clinically normal newborns. J Pediatr Orthop 1992;12:423–7.
Wood MK, Conboy V, Benson MKD. Does early treatment by abduction splintage improve the development of dysplasic but stable hips? J Pediatr Orthop2000;20:302–5.
Rosendahl K, Markestad T, Lie RT. Developmental dysplasia of the hip. A population based comparison of ultrasound and clinical findings. Acta Paediatr1996;85:64–9.
Tonnis D. Normal value of the hip joint for the evaluation of X-rays in children and adults. Clin Orthop Relat Res 1976;119:39–47.
Patel H. Preventative health care: 2001 update: screening and management of developmental dysplasia of the hip in newborns. CMAJ 2001;164:1667–77.
Shipman SA, Hefand M, Moyer VA, et al. Screening for developmental dysplasia of the hip: as systemic literature review for the US Preventative Services Task Force. Pediatrics 2006;117:e557–76.
Klisic PJ. Congenital dislocation of the hip—a misleading term: brief report. J Bone Joint Surg 1989;71:136.
Bialik V, Bialik GM, Blazer S, et al. Developmental dysplasia of the hip: a new approach to incidence. Pediatrics 1999;103:93–9.
Bache CE, Clegg J, Herron M. Risk factors for developmental dysplasia of the hip: ultrasonographic findings in the neonatal period. J Pediatr Orthop B2002;11:212–18.
Standing Medical Advisory Committee (SMAC). Screening for the detection of congenital dislocation of the hip. London: Department of Health and Social Security, 1986.
Jones D. Neonatal detection of developmental dysplasia of the hip. J Bone Joint Surg [Br] 1998;80:943–5.
Barlow T. Early diagnosis and treatment of congenital dislocation of the hip. J Bone Joint Surg [Br] 1962;44-B:292–301.
Macnicol MF. Results of a 25 year screening programme for neonatal hip instability. J Bone Joint Surg [Br] 1990;72:1057–60.
Editorial. Screening for congenital hip dysplasia. Lancet 1991;337:947–8.
Furnes O, Lie SA, Espehaug B, et al. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary hip replacements reported to the Norwegian Arthroplasty Register 1987–99. J Bone Joint Surg [Br] 2001;83:579–86.
Palmén K. Preluxation of the hip joint: diagnosis and treatment in the newborn and the diagnosis of congenital dislocation of the hip joint in Sweden during the years 1948–1960. Acta Paediatr 1961;50(Suppl 129):1–71.
Rosendahl K, Markestad T, Lie RT. Developmental dysplasia of the hip: prevalence based on ultrasound diagnosis. Pediatr Radiol 1996;26:635–9.
Bialik V, Wiener F. Sonography of suspected developmental dysplasia of the hip: a description of 3624 hips. J Pediatr Orthop 1993;2:152–5.
Green NE, Griffin PP. Hip dysplasia associated with abduction contracture of the contralateral hip. J Bone Joint Surg [Am] 1982;64:1273–81.
Wynne-Davies R. Acetabular dysplasia and familial joint laxity: Two etiological factors in congenital dislocation of the hip. J Bone Joint Surg 1970;52-B:704–16.
Good C, Walker G. The hip in the moulded baby syndrome. J Bone Joint Surg [Br] 1984;66:491–2.
Buxton RA, Macnicol MF. Infantile skeletal skew: the use of ultrasound in management. J Pediatr Orthop B 2004;13:75–80.
Castelein RM, Korte J. Limited hip abduction in the infant. J Pediatr Orthop 2001;21:668–70.
Newborn and Infant Physical Examination (NIPE). http:/nipe.screening.nhs.uk
Duppe H, Danielsson LG. Screening of neonatal instability and of developmental dislocation of the hip. A Survey of 132,601 Living newborn infants between 1956 & 1999. J Bone Joint Surg [Br] 2002;84-B:878–85.


