A New Technique for Fast and Safe Collection of Urine in Newborns
María Luisa Herreros Fernández, Noelia González Merino, Alfredo Tagarro García, Beatriz Pérez Seoane, María de la Serna Martínez, María Teresa Contreras Abad, Araceli García-Pose,
Arch Dis Child. 2013;98(1):27-29.
Abstract and Introduction
Abstract
Aim To describe and test a new technique to obtain midstream urine samples in newborns.
Design and methods This was a prospective feasibility and safety study conducted in the neonatal unit of University Infanta Sofía Hospital, Madrid. A new technique based on bladder and lumbar stimulation manoeuvres was tested over a period of 4 months in 80 admitted patients aged less than 30 days. The main variable was the success rate in obtaining a midstream urine sample within 5 min. Secondary variables were time to obtain the sample and complications.
Results This technique was successful in 86.3% of infants. Median time to sample collection was 45 s (IQR 30). No complications other than controlled crying were observed.
Conclusions A new, quick and safe technique with a high success rate is described, whereby the discomfort and waste of time usually associated with bag collection methods can be avoided.
Introduction
Clean urine samples are necessary to accurately diagnose several diseases in infants, especially urinary tract infections (UTIs). A wide range of clinical interventions for urine collection is described in the literature, including non-invasive and invasive methods. The most common non-invasive technique is urine collection using sterile bags, which is associated with significant patient discomfort and contamination of samples. Obtaining a clean-catch urine sample is the recommended method for urine collection in children able to co-operate. However, in children lacking sphincter control, urine catch is more difficult and time-consuming and invasive methods (catheterisation and needle aspiration of urine from the bladder) are sometimes needed.[1,2]
There are some stimulation techniques that facilitate emptying of the bladder in situations of bladder dysfunction.[3] We hypothesised that the use of such methods in newborns could facilitate the collection of a clean-catch urine sample.
The aim of this study was to determine the success rate and safety of a new non-invasive technique to obtain clean-catch urine samples in newborns.
Methods
This was a prospective feasibility and safety study conducted in Infanta Sofía University Hospital, Madrid (a secondary centre, with a 16-bed neonatal unit, a 30-bed nursery unit and a 24-bed paediatric ward). The study was carried out over 4 months (January–April 2010). Patients consisted of 90 consecutively admitted infants aged under 30 days who needed a urine analysis according to their attending physician. Exclusion criteria were: (1) poor feeding, (2) dehydration, (3) need for an immediate sterile urine sample obtained by an invasive method or a clinical situation that did not allow time to obtain a midstream urine sample, (4) any medical condition that ruled out manipulation, and (5) drug administration prior to urine collection.
The clinical research ethics committee of La Paz University Hospital, Madrid, Spain, approved this study.
Technique
Two people (trained nurses and/or physicians) were needed to perform the procedure, and a third to measure the time taken. This technique involves a combination of fluid intake and non-invasive bladder stimulation manoeuvres.
The first step is either breast-feeding or providing formula intake appropriate to the age and weight of the newborn. In babies fed infant formula, 10 ml was provided on the first day of life, increasing to 10ml per day during the first week. From the second week onwards, 25 ml/kg were administered before the onset of stimulation. Twenty-five minutes after feeding, the infant's genitals were cleaned thoroughly with warm water and soap and dried with sterile gauze. A sterile collector was placed near the baby in order to avoid losing urine samples. Before performing the technique, we administered non-pharmacological analgesia, such as non-nutritive sucking or 2% sucrose syrup, to prevent/lessen crying.
The second step is to hold the baby under their armpits with their legs dangling. One examiner then starts bladder stimulation which consists of a gentle tapping in the suprapubic area at a frequency of 100 taps or blows per minute for 30 s.
The third step is stimulation of the lumbar paravertebral zone in the lower back with a light circular massage for 30 s.
Both stimulation manoeuvres are repeated until micturition starts, and a midstream urine sample can be caught in a sterile collector (figure 1). Success is defined as the collection of a sample within 5 min of starting the stimulation manoeuvres.
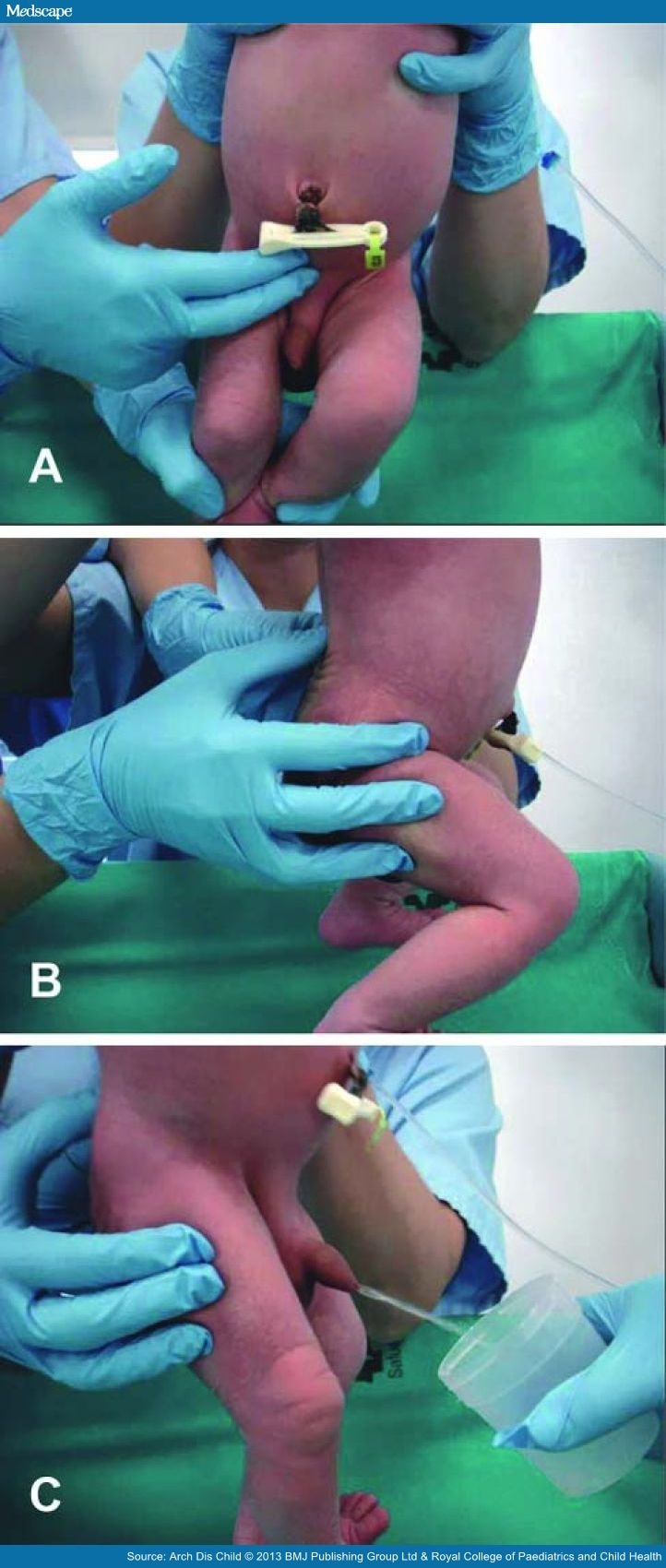
Figure 1.
New stimulation technique to obtain midstream urine in newborns. (A) Tapping in the suprapubic area. (B) Stimulation of the lower back. (C) Midstream urine sample collection in a sterile container.
Variables
The main variable was the success rate in obtaining a midstream urine sample within 5 min. Secondary variables were the time taken to obtain the sample and complications. The sample collection time was defined as the time from the beginning of the stimulation procedure (ie, the tapping) to the beginning of sample collection.
Analysis
We analysed the percentage of attempts where urine was obtained ('success'), the time to obtain the sample from the onset of bladder stimulation, age and sex. Categorical variables ('success', sex) are expressed as rates (%) and measurable variables (age, time) as mean (SD), 95% CI, median and IQR. Categorical variables were compared using Pearson's χ2 or Fisher's exact test. Quantitative variables were compared using Student's t test or by the Mann–Whitney U test. All statistical analyses were performed with SPSS software (V.17.0). We assumed significance at the 5% level (p<0.05).
Results
Ninety patients were eligible for the study. Indications for urine collection were neonatal jaundice (49), screening for infection (20), cytomegalovirus screening in low birthweight newborns (14) and renal pelvis dilatation (7). Ten patients were later excluded due to low oral intake leaving 31 girls and 49 boys. Their mean ages were 6.66 days for boys and 6.23 days for girls. There was an 86% success rate (n=69/80).
The mean time for sample collection was 57 s (SD 48.6), median 45 s and IQR 30 s. The mean time spent collecting the sample in males was 60.48 s, median 55 s and IQR 30 s. For females, the mean time was 52.04 s, median 30 s and IQR 30 s. Urine was sometimes obtained before the end of the first cycle of stimulation (<60 s).
No statistically representative differences with regard to sex were found in success rate, time of sample collection or complications. There were no complications, but controlled crying occurred in 100% of infants.
Discussion
We report a new technique to obtain a midstream clean urine sample in newborns, which has a high success rate and a mean time for passing urine of less than 1 min.
Midstream urine collection is the preferred method for adults and older children and is suitable for diagnosing UTI.[1] The collection of spontaneous urine in infants is also useful for UTI investigation, but is a time-consuming and unpredictable task that requires prolonged attention and patience, and so is not widely performed.
The advantages of midstream urine collection and the difficulties performing it in newborns encouraged us to develop a suitable technique for this age group. Invasive methods for obtaining clean urine (suprapubic aspiration and bladder catheterisation) are aggressive and have a high failure rate in newborns due to their anatomical characteristics and irregular voiding pattern.
We based the procedure on manoeuvres described for patients with bladder dysfunction to stimulate bladder emptying through reflex contraction of the detrusor muscle.[3] The detrusor muscle is innervated by the parasympathetic pelvic nerves (S2–S4). The spinal micturition reflex is a simple arch reflex. Distended bladder walls stimulate efferent fibres going to the medulla, the arch reflex is produced in S2–S4, and afferent fibres stimulate the detrusor muscle which contracts to pass urine. This reflex is voluntarily inhibited and controlled in continent individuals by the cortex, but not in newborns. In neonates, it can be triggered, as we propose.
We designed a stimulation technique and protocol and performed a study after nurses and physicians had been trained. We have demonstrated that this technique is effective in obtaining a urine sample in a majority of patients in an easy, safe and fast way. Bag changes, long waiting times and invasive techniques were avoided.
Limitations of this study include the lack of control group. Nevertheless, the time to obtain urine in other published series is much longer.[4] Several factors may have influenced the efficiency of our technique: trained staff performed the procedure and a standardised fluid intake favoured the onset of urination after 20–30 min in this age group.[5]
As far as we know, there is no other standardised method to facilitate micturition in infants. This technique has replaced the use of collection bags as a routine method for newborns in our centre. Other invasive and aggressive methods carrying some risk (suprapubic aspiration or bladder catheterisations) were also avoided.
Conclusions
A new method to obtain midstream urine in newborns is described. It consists of feeding, bladder stimulation and paravertebral lumbar massage. The technique has been demonstrated to be safe, quick and effective. The discomfort and waste of time usually associated with bag collection methods can be avoided, as well as invasive techniques.